Simulations help understand adsorption of molecules on α-alumina surface
23-01-2024
α-alumina, one of the forms of aluminum oxide, is a widely used ceramic material, partly for its adsorption capacity. The understanding of this mechanism has been advanced by recent studies using state-of-the-art DFT calculations carried out by NOMATEN scientists at the NCBJ, which were highlighted on the cover of a special issue of the journal Physica Status Solidi B.
The most common aluminium oxide is Al2O3, especially one of its natural forms – corundum. In addition to the gem varieties ruby and sapphire, this oxide has also found wide use as a ceramic material due to its properties. α-alumina, as the compound is called in materials science, is resistant to high temperatures, radiation damage and corrosion. Among other things, it is used as a catalyst in certain chemical reactions and as an adsorbent, given its very good ability to bind particles on its surface, such as water molecules (it acts as a moisture absorber or filter in water treatment plants). Moreover, the addition of other metals to the compound may allow it to effectively break down water molecules and, as a result, produce hydrogen, which currently is a topic of increasing interest.
Performing studies of the adsorption of molecules on α-alumina is a complex task. The material’s complex crystalline structure and surface defects make atomic simulations very important for understanding the mechanisms at work. The DFT (density functional theory) method, which is often used in this case, is capable of accurately modelling the structures under investigation, but this involves the use of very large computational resources, especially when modelling structures consisting of different atoms. For this reason, the researchers of the NOMATEN Centre of Excellence at NCBJ decided to use the SCC-DFTB (self-consistent-charge density-functional tight-binding) approach in their work ’Dynamical Pathways for the Interaction of O2, H2O, CH4, and CO2 with α-Alumina Surfaces: Density-Functional Tight-Binding Calculations’. „This method is a combination of classical approaches and electron structure theory, while requiring far fewer computational resources than classical DFT calculations”, says NOMATEN’s dr hab. Javier Dominguez, first author of the paper. „It is now a versatile tool in both materials research and chemistry thanks to its ability to model large structures over longer timescales than with ordinary DFT. This allows us to analyse the dynamic properties of materials. In the current research, the use of this method has allowed us to model the mechanisms of dissociation and molecule formation based on the interactions of various atoms with aluminium and oxygen atoms on the studied surface.”
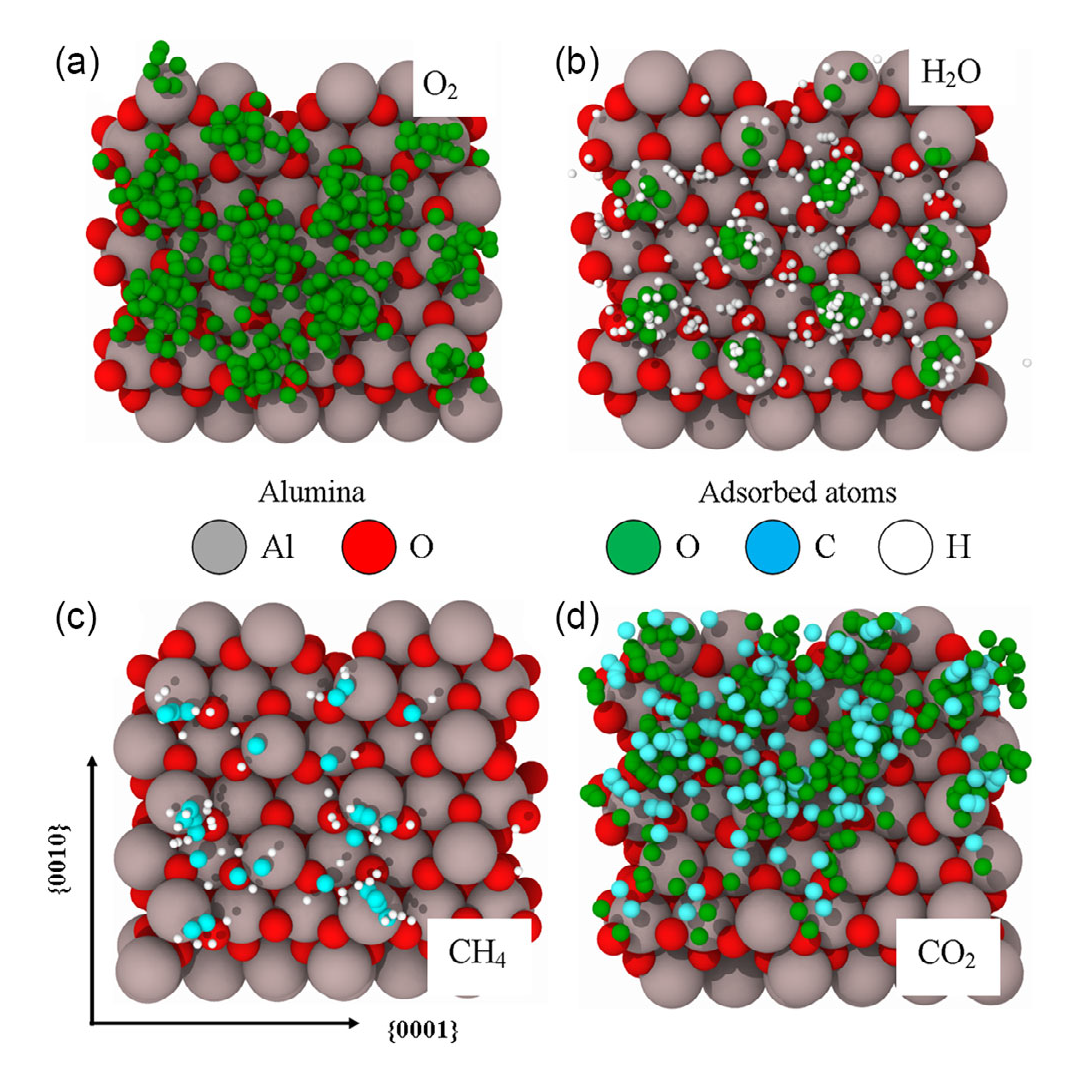
The subject of the research by the group from the NOMATEN Centre of Excellence Was the interactions of the α-Al2O3 surface with various molecules – oxygen O2, water H2O, methane CH4 and carbon dioxide CO2. Calculations using DFTB methods, supported by quantum-classical molecular dynamics (QCMD) simulations for room temperature, were intended to help understand the mechanisms of interaction of this material with molecules, which is essential in view of their use in the design and optimisation of, for example, hydrogen production or sensors development.
Given the structure of the material studied, the researchers considered two cases – surfaces with aluminium atoms on top (Al-terminated) and surfaces with oxygen atoms on top (Ox-terminated). These cases differed, among other things, in the stability or distribution of the adsorption areas. Similarly, the orientation of the molecules relative to the surface Was taken into account – molecules arranged perpendicularly and parallel behaved differently. „Oxygen molecules showed the highest adsorption, while methane molecules showed the lowest,” describes Javier Dominguez. „We also found that CH4 shows the ability to dissociate on the surface of α-alumina, the product of which is atomic or molecular hydrogen. It is therefore possible that this mechanism could be used to produce hydrogen from methane. In addition, simulations also showed an increase in the optical adsorption of alumina, especially in the visible spectrum.”
The results obtained in the simulations agree with both experiments and theoretical values. The NOMATEN researchers’ study has shown that the SCC-DFTB method can be successfully used to model adsorption mechanisms on the α-alumina surface. This has already been recognised by the scientific community and the work Was honoured with a place on the cover of a special issue of the journal Physica Status Solidi B.
Original paper:
Domínguez-Gutiérrez, F.J., Aligayev, A., Huo, W., Chourashiya, M., Xu, Q. and Papanikolaou, S (2024), Dynamical Pathways for the Interaction of O2, H2O, CH4, and CO2 with α-Alumina Surfaces: Density-Functional Tight-Binding Calculations. Phys. Status Solidi B, 261: 2200567. https://doi.org/10.1002/pssb.202200567





